ADC Therapeutics Announces First Patient Dosed in Phase I Clinical Trial of ADCT-601 in Advanced Solid Tumors
Pyrrolobenzodiazepine-based antibody drug conjugate targets AXL, a receptor tyrosine kinase highly expressed in solid tumors
LAUSANNE, Switzerland, Jan. 16, 2019 (GLOBE NEWSWIRE) — ADC Therapeutics, an oncology drug discovery and development company that specializes in the development of proprietary antibody drug conjugates (ADCs), today announced that the first patient has been dosed in its Phase I clinical trial evaluating the safety, tolerability, pharmacokinetics and anti-tumor activity of ADCT-601 in patients with selected solid tumors that are locally advanced or metastatic.
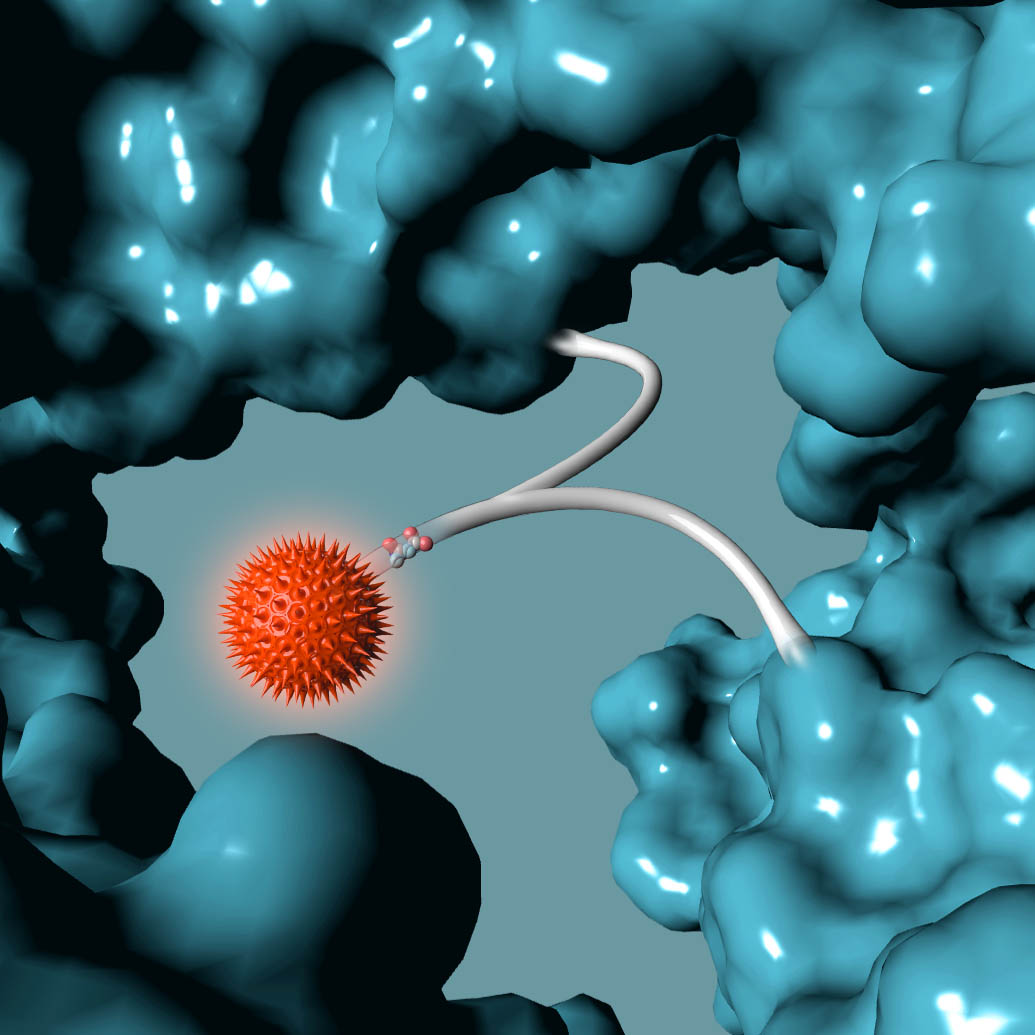
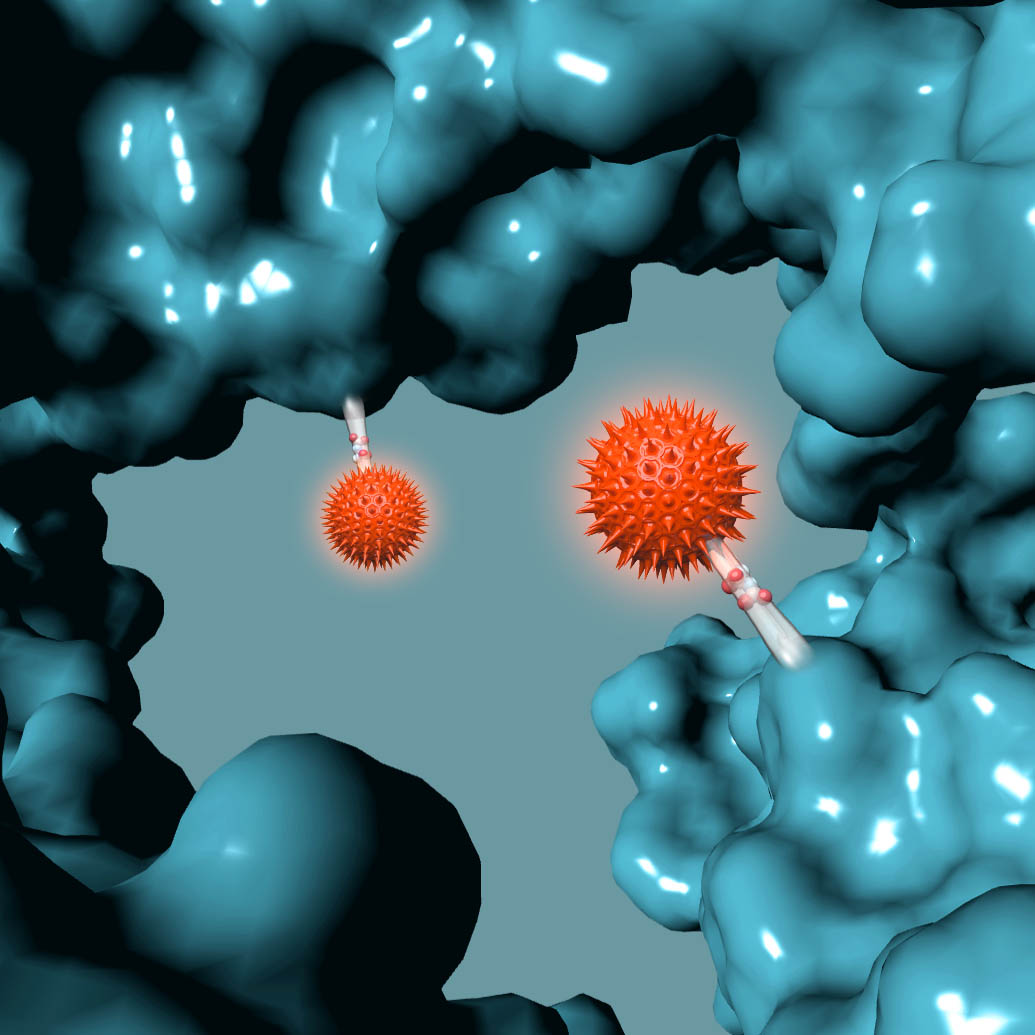
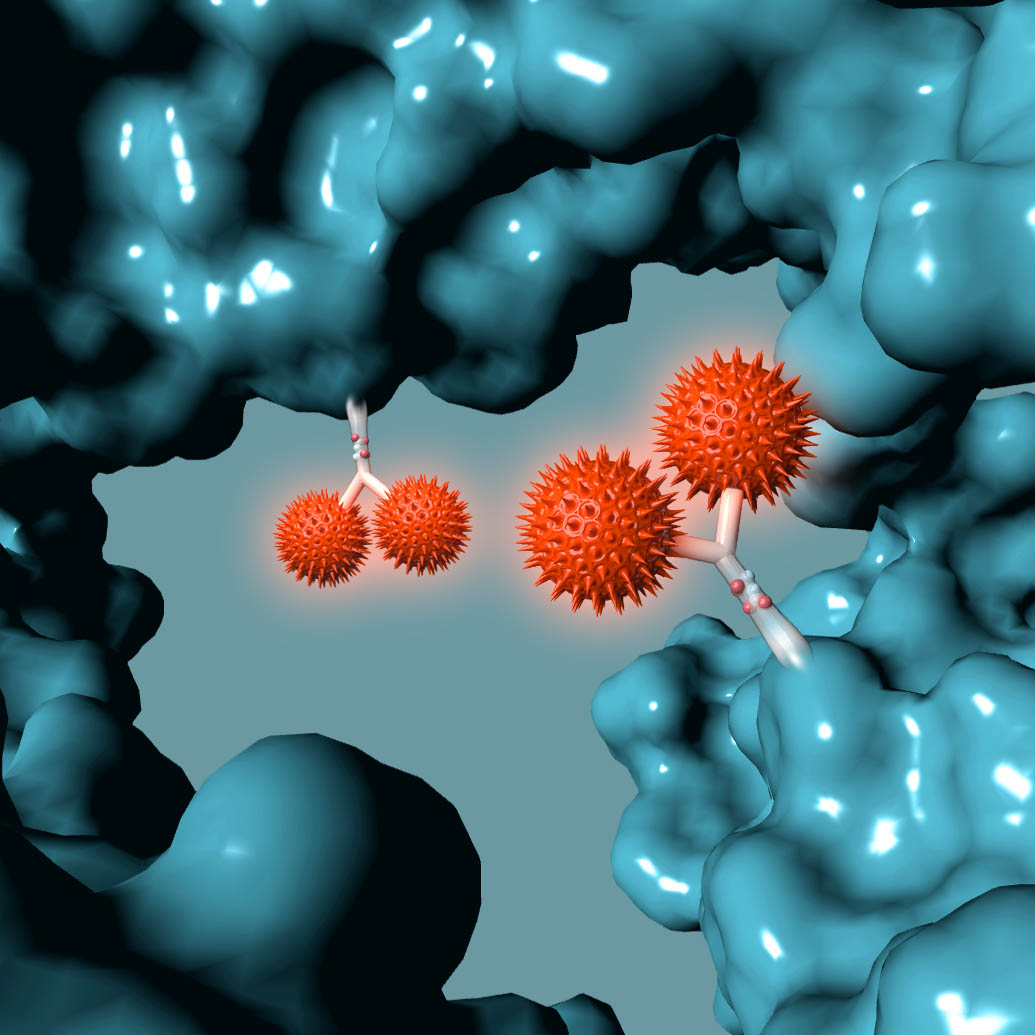
ADCT-601 is an ADC composed of a humanized monoclonal antibody against human AXL, conjugated using GlycoConnect™ site specific conjugation technology to a pyrrolobenzodiazepine (PBD) dimer toxin. In preclinical studies, ADCT-601 demonstrated potent and specific in vitro and in vivo anti-tumor activity in multiple cancer-derived models with different levels of AXL expression, and was stable and well tolerated.
Jay Feingold, MD, PhD, Chief Medical Officer and Senior Vice President of Clinical Development at ADC Therapeutics, said, “AXL is a novel and ideal target for an ADC approach, as it is overexpressed in many solid tumor types. We look forward to exploring the effect of ADCT-601 on patients with selected advanced solid tumors who have failed or are intolerant to any established therapy. With five ADCs in eight ongoing clinical trials for multiple indications, we believe our highly targeted therapies have the potential to meaningfully improve outcomes for patients with solid tumors and hematological cancers.”
The open-label, multicenter, single-arm trial will include a Phase Ia dose-escalation part followed by a Phase Ib dose-expansion part. The dose-escalation part is designed to determine the maximum tolerated dose of ADCT-601. The identified dose will be evaluated in the dose-expansion part. Approximately 75 patients will be enrolled in the trial. For more information, please visit www.clinicaltrials.gov (identifier NCT03700294).
About ADCT-601
ADCT-601 is an antibody drug conjugate (ADC) composed of a humanized monoclonal antibody that binds to human AXL, conjugated using GlycoConnect™ technology to a linker with a pyrrolobenzodiazepine (PBD) dimer toxin. Once bound to an AXL-expressing cell, ADCT-601 is internalized into the cell where enzymes release the PBD-based warhead. The PBD-based warhead has the ability to form highly cytotoxic DNA interstrand cross-links, blocking cell division and ultimately killing the cancer cell. ADCT-601 is being evaluated in a Phase I clinical trial in patients with advanced solid tumors (NCT03700294).
About ADC Therapeutics
ADC Therapeutics SA is an oncology drug discovery and development company that specializes in the development of proprietary antibody drug conjugates (ADCs) targeting major hematological malignancies and solid tumors. The Company’s ADCs are highly targeted biopharmaceutical drugs that combine monoclonal antibodies specific to surface antigens present on particular tumor cells with a novel class of highly potent pyrrolobenzodiazepine (PBD)-based warheads via a chemical linker. The Company has five PBD-based ADCs in ongoing clinical trials, ranging from first in human to pivotal Phase II, in the USA and Europe, and a deep pipeline of other preclinical ADCs in development. ADC Therapeutics has world-class partners, including AstraZeneca and its global biologics research and development arm, MedImmune. The Company is based in Lausanne (Biopôle), Switzerland and has operations in London, San Francisco and New Jersey. For more information, visit www.adctherapeutics.com.