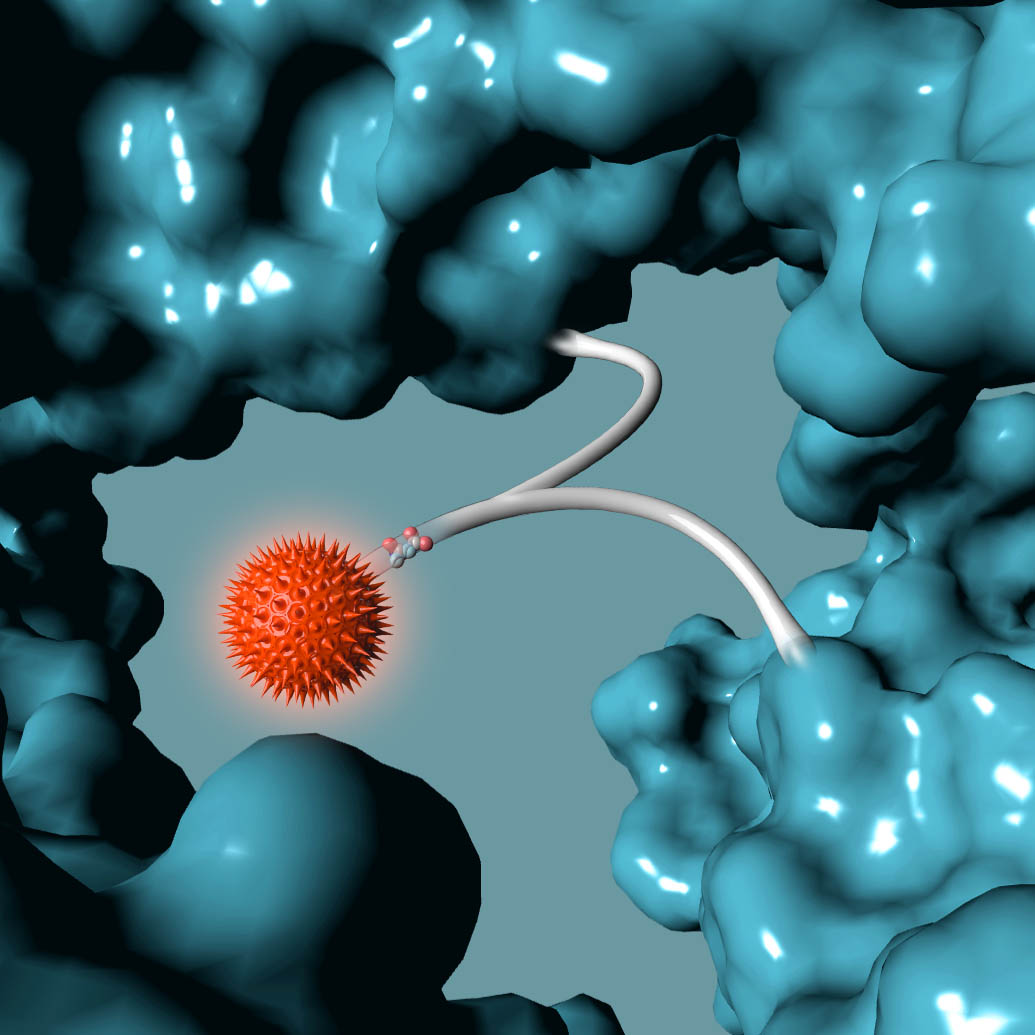
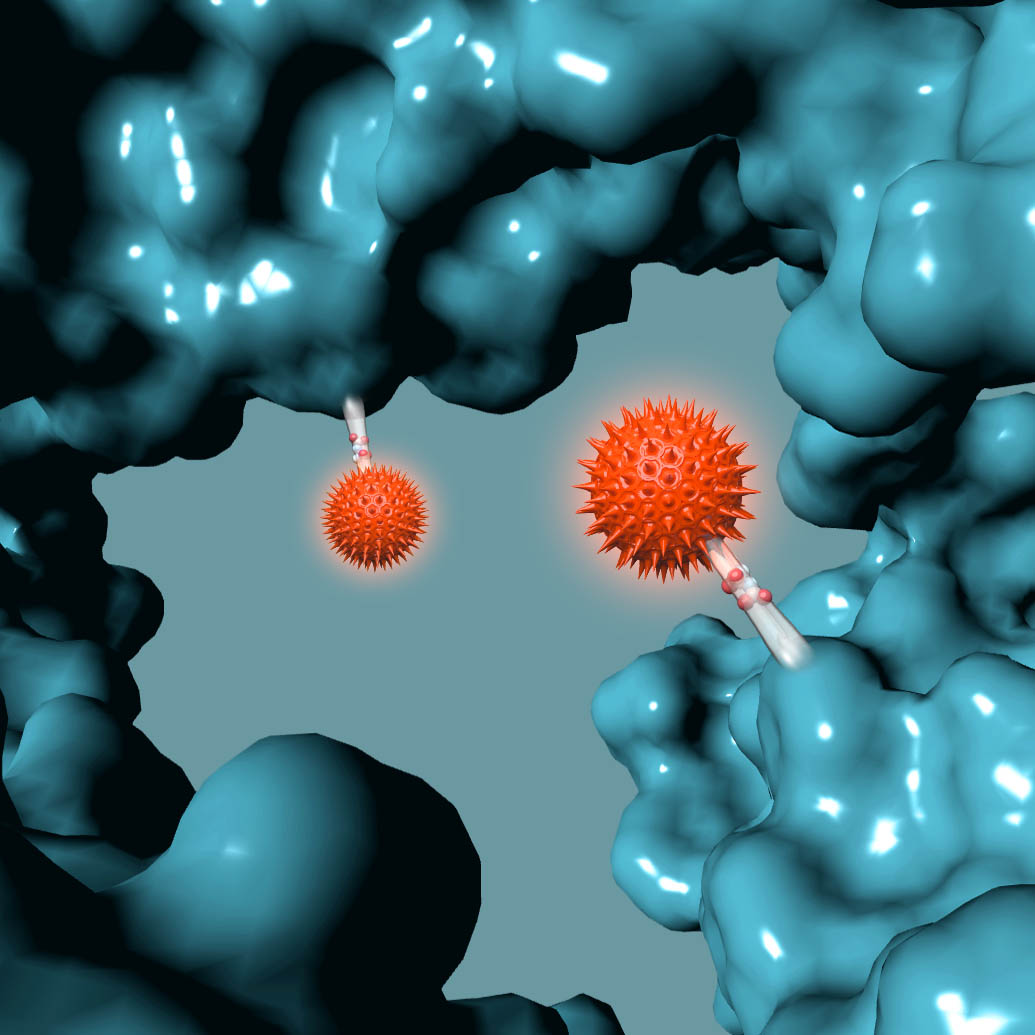
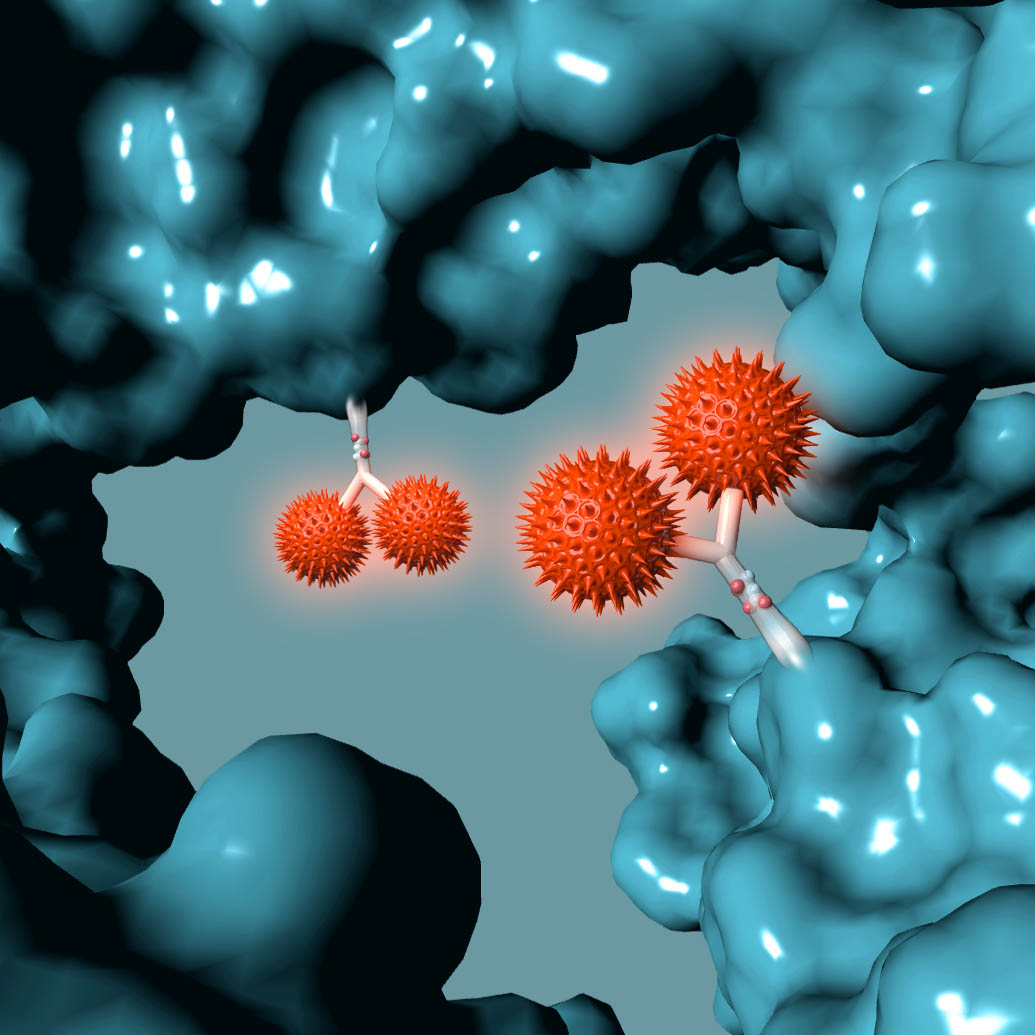
AMSTERDAM, NETHERLANDS – October 20, 2016 – Synaffix BV announced today it has entered into a Commercial License Agreement with ADC Therapeutics for its proprietary GlycoConnect™ and HydraSpace™ site-specific antibody-drug conjugate technologies.
Under the terms of the agreement, ADC Therapeutics has been granted a single-target license for one of its preclinical programs and has also been granted an option to take a limited number of additional single-target licenses for potential future programs.
Floris van Delft, CSO at Synaffix said,
“We are delighted that ADC Therapeutics has recognized the value of our proprietary antibody-drug conjugate technologies and has elected to incorporate Synaffix technology into one of its preclinical programs.”
“The experience of Synaffix and its partners has consistently confirmed that, in preclinical models, our proprietary GlycoConnect™ and HydraSpace™ technologies significantly improved both efficacy and safety as compared to other mainstream site-specific conjugation approaches.”
“We look forward to working closely with the ADC Therapeutics team to advance these promising therapeutics to the patients who need them.”
Synaffix is eligible to receive upfront, milestone and royalty payments on a per-target basis.
About GlycoConnect™ and HydraSpace™
The Synaffix technology platforms include GlycoConnect™, the site-specific and stable antibody conjugation technology that involves proprietary enzymes and metal-free click conjugation reagents, and HydraSpace™, the antibody-drug conjugate enhancing spacer technology.
GlycoConnect™ was shown to be capable of significantly enhancing the therapeutic index of an antibody-drug conjugate on its own. The highly polar properties of HydraSpace™ improve the solubility and stability of the payload and the resulting antibody-drug conjugate product, thus enhancing further the therapeutic index of the antibody-drug conjugate.
Both technologies have demonstrated compatibility with all antibody-drug conjugate payload classes and all IgG isotypes without requiring antibody engineering.
About Synaffix BV
Founded in 2010, Synaffix BV is a Netherlands-based biotechnology company exclusively focused on the continued advancement of best-in-class ADC technology platforms. As a leading innovator in the field of ADCs offering absolute versatility and state-of-the-art solutions, our vision is to become the preferred partner in the development of these complex biological therapeutics and realize our ambition – connect to cure™. Synaffix is backed by a top tier, life science-focused investor syndicate including Aravis, BioGeneration Ventures, BOM Capital and Merck Ventures, the strategic corporate venture capital fund of Merck. For more information, please visit the website at www.synaffix.com.
Synaffix BV Contact
Anthony DeBoer
Director, Business Development
Tel: +31 620 773 194